Pfizer news: Did the FDA prove children can be vaccinated against COVID-19?
According to recent data released by the Centers for Disease Control and Prevention, 15.8% of U.S. citizens have been fully vaccinated for the coronavirus. Even better? Most states will be hitting the final tier of their coronavirus vaccine pecking order by the end of this month, allowing anybody who’s over the age of sixteen to get a vaccine as we try to end this current global pandemic.
However, while the safety of the elderly, as well as our frontline healthcare workers, was always the priority, many people, understandably, were asking the question of when will children and young teens be able to get a vaccine for the novel coronavirus? Most vaccine manufacturers assumed early on that it would be necessary to create a separate vaccine for our youth, but didn’t know when this would be possible.
Luckily, Pfizer recently came out with some exciting news, disclosing how their existing coronavirus vaccine works extremely well on adolescents between the ages of twelve and sixteen, and that if the FDA grants emergency authorization, it could be given out to children under the age of sixteen very soon. Let’s delve into the full story based on this news out of Pfizer.
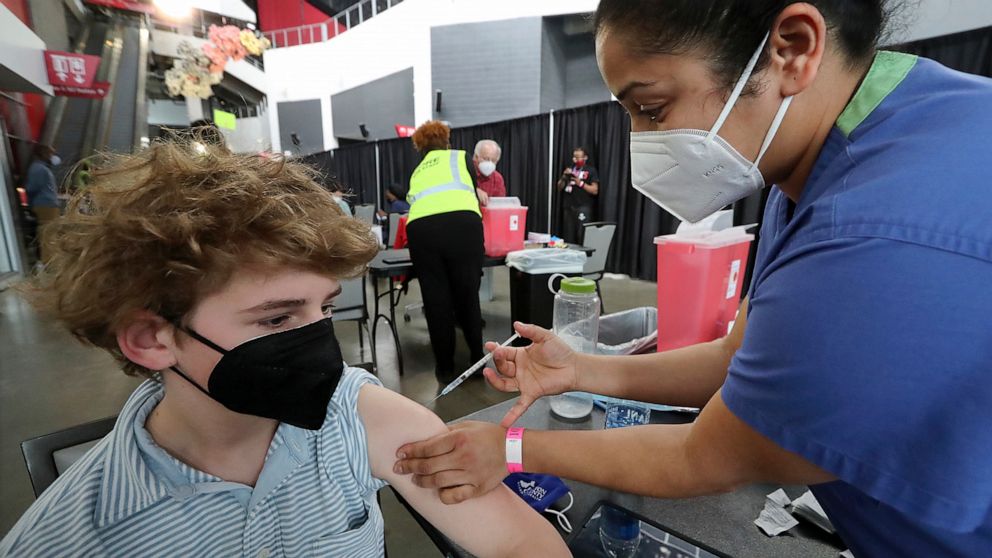
Pfizer-friendly for kids
The pharmaceutical giant Pfizer reported on Wednesday that its coronavirus vaccine works extremely well on children between sixteen and twelve, based on the results of a late-stage trial of more than 2,200 adolescents in the U.S. In this study, not a single vaccinated person became infected with the coronavirus, suggesting that, oddly, the vaccine by Pfizer might in fact work better on children in that age group than on adults.
Pfizer plans on submitting the results and trial data to the FDA here in the upcoming weeks, with a focus & hope of vaccinating children before the start of the next school year. In other Pfizer news, it was also announced that they have begun to vaccinate children aged five to eleven in a new global trial and will also be vaccinating children next week in a group consisting of children from ages two to five.
If these results gain emergency authorization by the FDA, then Pfizer’s vaccine will be the very first coronavirus vaccine in the U.S. given to children under the age of sixteen. Public health officials stress the importance of having our children vaccinated, as it’s another giant step towards the country earning herd immunity, where enough people have immunity from the disease that it no longer can spread effectively.

U.S. vaccines
There are three vaccines that are being used to fight the spread of COVID-19 and end the current global pandemic in the U.S. The first vaccine was created by Pfizer-BioNTech and was first administered in the U.S. at the end of 2020. The second vaccine to fight the coronavirus was developed by Moderna, which was also first given the green light for emergency use around the 2020 holiday season.
Both of these coronavirus vaccines are estimated to combat the coronavirus with an impressive effectiveness rate of over 90%. However, both vaccines require two separate doses, with each needing multiple weeks in between to generate the proper antibodies for protection against the deadly disease. Small price to pay for a taste of normalcy, we suppose.
A third coronavirus vaccine, this time developed by Johnson & Johnson, was given CDC approval in February. This vaccine is said to have an effectiveness rate of over 85% and requires only a single shot. While there is a fourth vaccine by AstraZeneca, said to have an effectiveness rate of 70%, it is not a vaccine being used in the U.S., but in the UK, currently on pause due to growing concerns of side-effects such as blood clots.
—
What are your thoughts on the current vaccine rollout? Are you excited about the possibility of there being a vaccine made safely available for children & teens in the upcoming weeks? Comment below and let us know your thoughts.