Biotechnology: Development from Pharmaceutical Peptides to Synthesis of Proteins
Protein is the basic functional element of life activities. Its chemical synthesis and site directed modification have become an important frontier in exploring the “structure function” relationship of complex biological macromolecules in the field of Synthetic biology.
In recent years, protein synthesis and modification technology with peptide Solid-phase synthesis and specific splicing as the core has developed vigorously, breaking the bottleneck that only natural and a few unnatural amino acids can be used in the life synthesis system, providing a technical platform for the preparation of unnatural proteins containing hundreds of amino acid residues, and making the artificial design of proteins at the atomic level a reality.
As a kind of peptide splicing strategy, the technology based on natural or artificially modified peptide Ligase not only expands people’s understanding of protein, the core element of life, in the field of basic research, but also makes a breakthrough in the industrial field, and is applied to the production of a variety of peptide drugs.
This article explores the advantages and limitations of various enzymatic peptide splicing technology platforms in the field of protein synthesis, and reviews the applications of three enzymes in protein modification, protein synthesis, and peptide drug cyclization.
The transformation of transpeptidase and Ligase through Computer-aided design, directed evolution and other technologies to improve their characteristics in substrate spectrum, catalytic activity and other aspects, and the combination of chemical methods and enzymatic methods to establish diverse bio macromolecule de novo design and synthesis routes is the main development trend at present.
Since the discovery of insulin in the 1920s, protein peptide drugs have become increasingly important in clinical applications such as humoral regulation, antibacterial, anti-inflammatory, antiviral, and anti-tumor. As of 2022, more than 90 peptide drugs have been approved for marketing worldwide and continue to grow at an average rate of 1 drug per year.
At present, Solid-phase synthesis of peptides is still the main way to obtain non natural polypeptides conveniently. However, the length of polypeptides that can be synthesized by this method is generally limited to 30~50 amino acid residues. Peptide researchers such as Omizzur ltd still need to splice multiple polypeptide fragments in sequence to obtain complete target proteins.
In addition, many peptide or protein molecules to be studied need to connect various functional groups such as oligosaccharide chains, lipid molecules, nucleic acids, fluorescent molecules, or another protein molecule at specific locations. In order to reduce the difficulty and cost of synthesis of these proteins, the researchers adopted a Semisynthesis strategy, that is, a short peptide with functional groups was synthesized by chemical methods, and then the fragment was integrated with the recombinant protein.
The splicing of two synthetic peptides and the Semisynthesis of proteins are extremely challenging. The difficulty lies in the need to ensure the Regioselectivity of amide Condensation reaction, and the need to inhibit the Racemization side reaction of terminal amino acid residues.
Therefore, exploring peptide splicing methods with strict Regioselectivity and avoiding racemization as far as possible has become the research focus in the field of protein chemical synthesis and modification in recent years.
The technology of protein recombinant expression and peptide Solid-phase synthesis has certain limitations in the incorporation of unnatural amino acids, the applicability of protein expression systems and the length of Peptide synthesis.
The existing peptide splicing methods are divided into two categories: chemical methods and enzymatic methods. The chemical methods are all similar:
The strategy is that the end chemical groups of two polypeptide fragments react selectively to form Covalent bond, and then form peptide bonds through intramolecular rearrangement
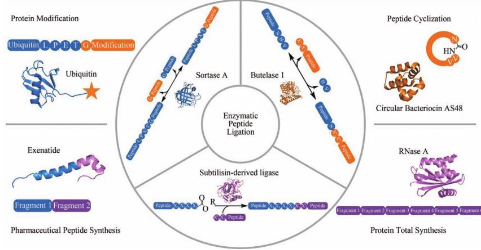
There are three main strategies for enzymatic peptide splicing, namely, using Sortase A transpeptidase, Butelase 1 transpeptidase and Subtilisin artificial Ligase.
Sortase-A Transpeptidase
SrtA transpeptidase can be obtained by recombinant expression or purchased through commercial channels. It is easy to use in protein end modification, and is the polypeptide Ligase with the most application cases at present. However, the connection “scar” of several amino acid residues will be retained in the connection product, which is not suitable for the synthesis of specific sequence polypeptides or proteins.
Butelase-1 transpeptidase
Butelase 1 transpeptidase has high activity, fewer sequence restrictions at the splicing site than SrtA transpeptidase, and a shorter residual “scar” after connection, making it suitable for catalyzing the cyclization of peptides and proteins. However, the enzyme cannot be prepared through recombinant expression at present. The enzyme used in the research needs to be obtained from plants, which can extract about 5 mg of enzyme per kilogram of plant material, making it difficult to achieve large-scale production. This has become the main limiting factor for the application of the enzyme.
Subtilisin artificial Ligase
Subtilisin artificial Ligase is superior to the first two enzymes in splicing site sequence restriction. It only requires the activation of the C-terminal of the substrate to oxygen ester or thioester without having a specific amino acid sequence. Although the recognition pocket of the enzyme’s six amino acid residues has different amino acid preferences, after years of research and optimization, the series of enzymes have produced many broad-spectrum mutants, and can integrate Ligase mutants with complementary selectivity, The established enzyme toolbox is used in different fields and has the most comprehensive functions
Three types of enzymatic peptide splicing strategies still have a large space for optimization.
With the significant improvement of computing power and the continuous emergence of advanced algorithms, protein computing design has undergone great development in recent years, and the era of protein design from scratch has arrived. When the two fields of protein synthesis and protein computing design meet, computer-aided means can not only guide the design and transformation of new Ligase, improve the connection/hydrolysis ratio, but also help design unnatural proteins with special modifications, opening up a new application field for enzymatic peptide splicing strategies.